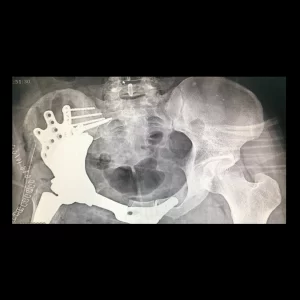
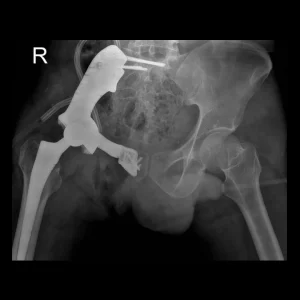
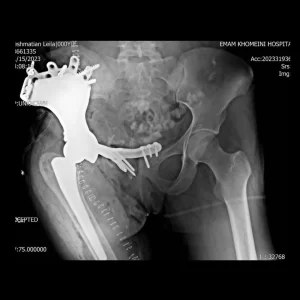
Custom Ankle Fusion Cages
Custom ankle cages are specialized implants designed for patients with talar avascular necrosis (AVN), tailored to each patient’s unique anatomy. These implants improve patients’ satisfaction with great surgical outcomes through the use of biocompatible medical-grade materials and cutting-edge 3D printing technology. The following conditions warrant the utilization of these cages: avascular talus necrosis, severe arthritis [1], traumatic fractures, and Charcot deformities [3].

Product Overview
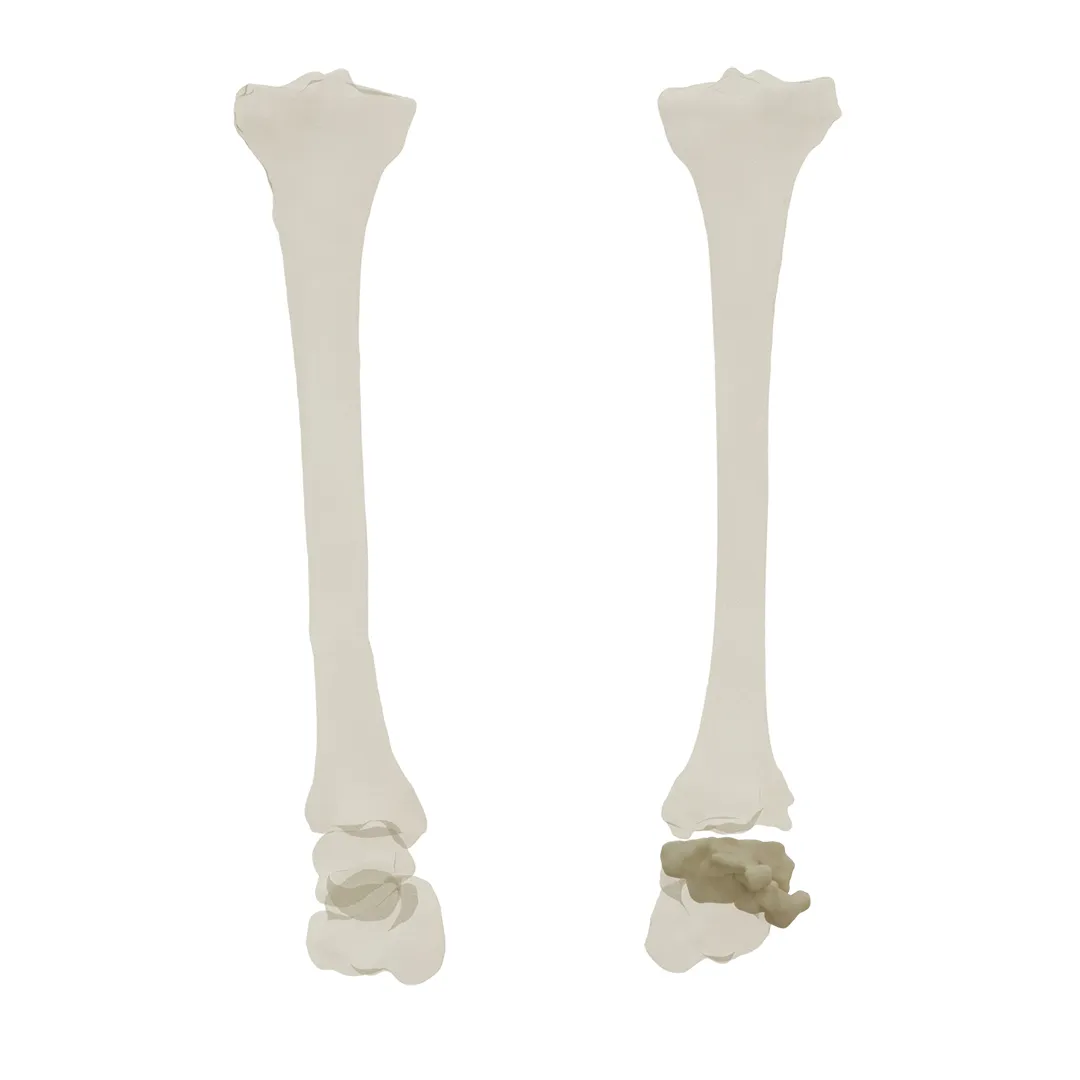
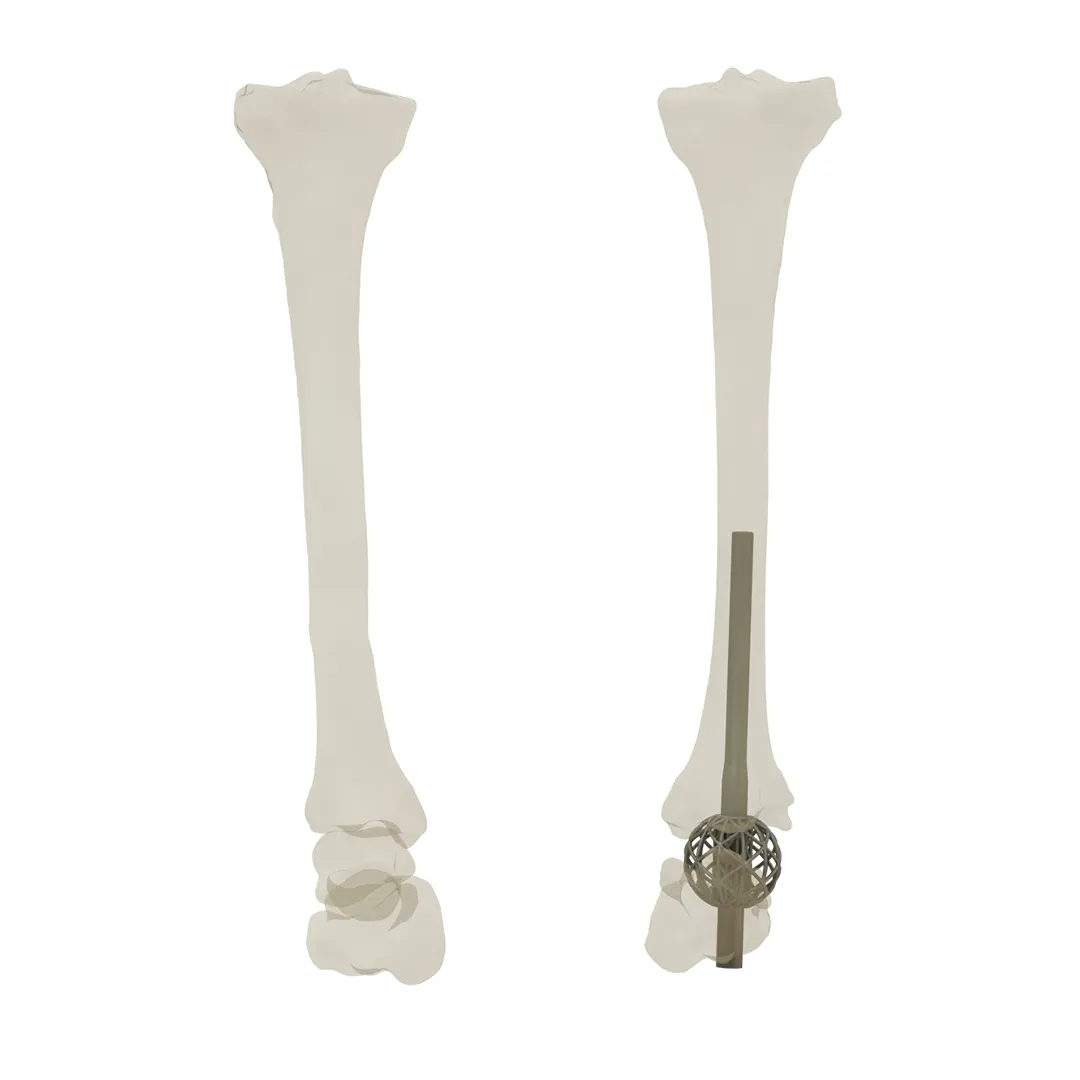
Product Overview
Since every patient’s anatomy is unique, off-the-shelf cages limit the surgeon’s choice for a treatment plan, while customized cages are created based on the physician’s ideal treatment plan, which results in higher-quality care for the patient.
In order to ensure optimal compliance with the patient’s anatomy and the physician’s treatment plan, custom ankle cages are designed based on patient CT scans, accounting for the possibility of larger bone necrosis during the design and manufacturing process.
The likelihood of a successful surgical result is greatly increased when biocompatible medical-grade materials and state-of-the-art 3D printing technology are used in the production of these cages with special porosity design based on surgeon’s preference.
For patients with avascular bone necrosis, Tibotalocalcaneal Arthrodesis [1] or Charcot ankle deformity [3]; these cages are the best option since they do away with the requirement for prefabricated, one-size-fits-all designs, which frequently necessitate alterations and adjustments to the cage and the fusion intramedullary nail and also limit the treatment plan options available to physicians.
Workflow
Imaging
To match the existing deficiency without the need for future bone reduction, a realistic 3D model of the patient's anatomy is generated using CT and Xray imaging.
Cage Design
Considering the patient's anatomy and soft tissue features, biomedical engineers create a tailored cage.
Product Manufacturing
3D printing technology is used to construct the cage with titanium, cobalt-chrome, and tantalum when a doctor gives permission to proceed.
Surgery
Anatomical model of the patient and the sterilized cage are given to the physician. An employee of our company may serve in the operating room to help with cage placement.
Specifications
Based on the clinical evaluation of the patient and the desired course of therapy, surgeons and the engineering team can work together to develop the shape and features of the cage.
In addition to intramedullary nails, to encourage bone ingrowth and osteointegration for the better secondary stability, the base structure of the spherical cage may incorporate a TPMS lattice structure, such as gyroid.
Personalized cages allow for the customization of any design according to the doctor’s preferences, and the inclusion of a truss-based lattice which is filled with autograft, reduces the possibility of non-union.
Based on the intended nail size used by the surgeon, we manufacture the intramedullary canal size.
We create modular cages that are tailored to the one-of-a-kind anatomy of the patients with the deformity or issue in their distal tibia. [2]
Specifications
Based on the clinical evaluation of the patient and the desired course of therapy, surgeons and the engineering team can work together to develop the shape and features of the cage.
benefits
- Short Stature Prevention
- Preventing graft resorption and infection
- Reducing Surgical Time and Costs
- Patient-Specific Implant Design for Bone Defects
- Porous Structure in Bone Implants: Enhancing Osseointegration and Fixation

Cases report
The patient is a 31-year-old man diagnosed with pelvic osteosarcoma, who presented with...
The patient is a 36-year-old man who has been diagnosed with pelvic sarcoma...
The patient is a 22-year-old woman who has been affected by a Giant...
The patient is a 49-year-old man who had previously undergone surgery and subsequently...
Cases report
Our case studies demonstrate the positive results that occur from using our specially designed devices. They illustrate the efficacy of our treatment programs and our team’s skill in developing individualized solutions for every patient. You may get a better idea of the amount of attention to detail and precision we apply to each and every area of our work with patient-specific implants by looking over our reports.